almaradiologie.be
Campus Eeklo Radiologie: 09/376.04.70 CT-scan: 09/376.04.79 NMR: 09/376.06.88 Fax:09/376.07.42 [email protected] CoronaroCT duur van het onderzoek: het onderzoek zelf inclusief het aanprikken, positioneren, en scannen duurt 10 - 15 minuten. Nadien dienen er door de radioloog nog uitgebreide sofwarematige bewerkingen en reconstructies van de beelden te Bepaling worden uitg
 Typical LA-MC-ICP-MS operating settings for Li isotope
Nickel‘‘wet’’ plasma sampler‘‘light-element high-abundance’’
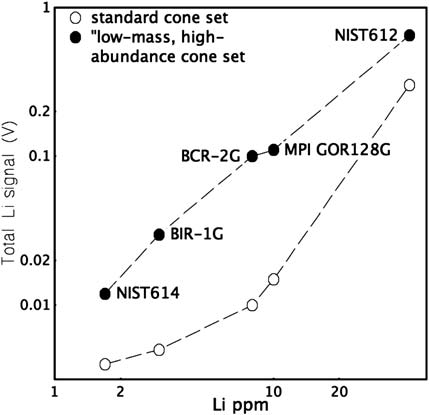
Total Li signal in volts obtained during analysis of the labelled
standard glasses using the standard NuPlasma ‘‘dry’’ plasma cone set and
the experimental ‘‘low-mass, high-abundance’’ cone set, versus Li
concentrations (ppm) of these standard glasses.
Typical LA-MC-ICP-MS operating settings for Li isotope
Nickel‘‘wet’’ plasma sampler‘‘light-element high-abundance’’
Total Li signal in volts obtained during analysis of the labelled
standard glasses using the standard NuPlasma ‘‘dry’’ plasma cone set and
the experimental ‘‘low-mass, high-abundance’’ cone set, versus Li
concentrations (ppm) of these standard glasses.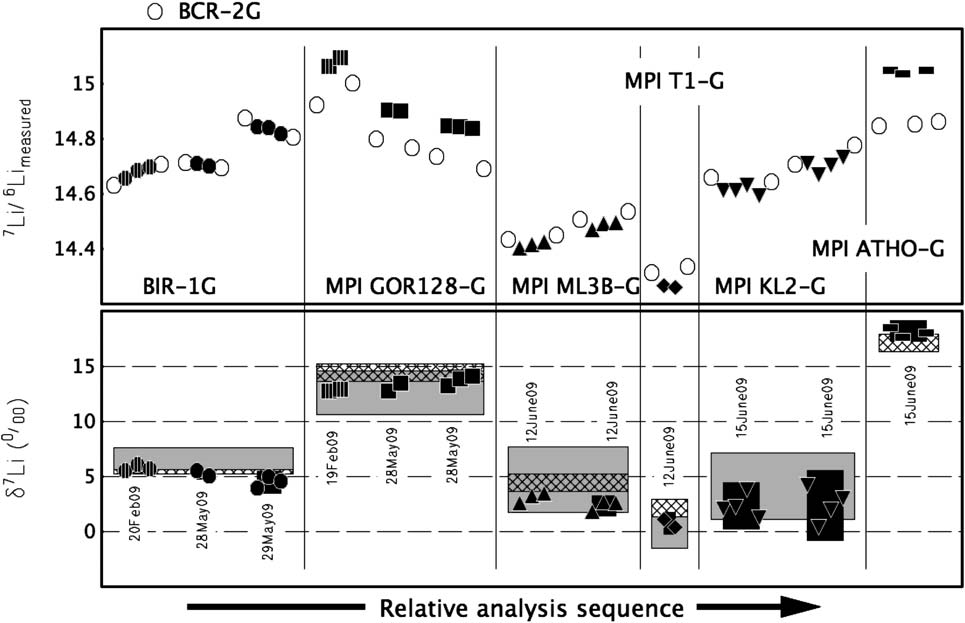 Top panel. Measured 7Li/6Li ratios of bracketing BCR-2G standard analyses and analyses of other standards as unknowns. Bottom panel. d7Li
(&) of labelled standards as measured in this study; corrected for instrumental drift, mass fractionation and referenced through BCR-2G to LSVEC(NIST 8545, 7Li/6Li 12.1716). Filled symbols individual analyses, black filled fields average Æ2s of each bracketed session, hatched fields publishedMC-ICP-MS and gray filled fields published SIMS values for each standard (see Table 2). Vertical-lined symbols data collected using standard cones, allother data collected using ‘‘low-mass, high-abundance’’ cone set. Date label indicates day of analysis.
Top panel. Measured 7Li/6Li ratios of bracketing BCR-2G standard analyses and analyses of other standards as unknowns. Bottom panel. d7Li
(&) of labelled standards as measured in this study; corrected for instrumental drift, mass fractionation and referenced through BCR-2G to LSVEC(NIST 8545, 7Li/6Li 12.1716). Filled symbols individual analyses, black filled fields average Æ2s of each bracketed session, hatched fields publishedMC-ICP-MS and gray filled fields published SIMS values for each standard (see Table 2). Vertical-lined symbols data collected using standard cones, allother data collected using ‘‘low-mass, high-abundance’’ cone set. Date label indicates day of analysis.