Paroxetine in social phobia
The Efficacy of Paxil (Paroxetine) for Panic Disorder Journal : The Current Practice of Medicine Authors : Dr Sean D. Hood, Dr Spilios Argyropoulos, Prof David J. Nutt Corresponding Author : Sean Hood ([email protected]) Introduction Substantial advances into the pharmacological treatment of Panic Disorder (PD) have been made in the lasttwo decades. Although tricyclic antidepres
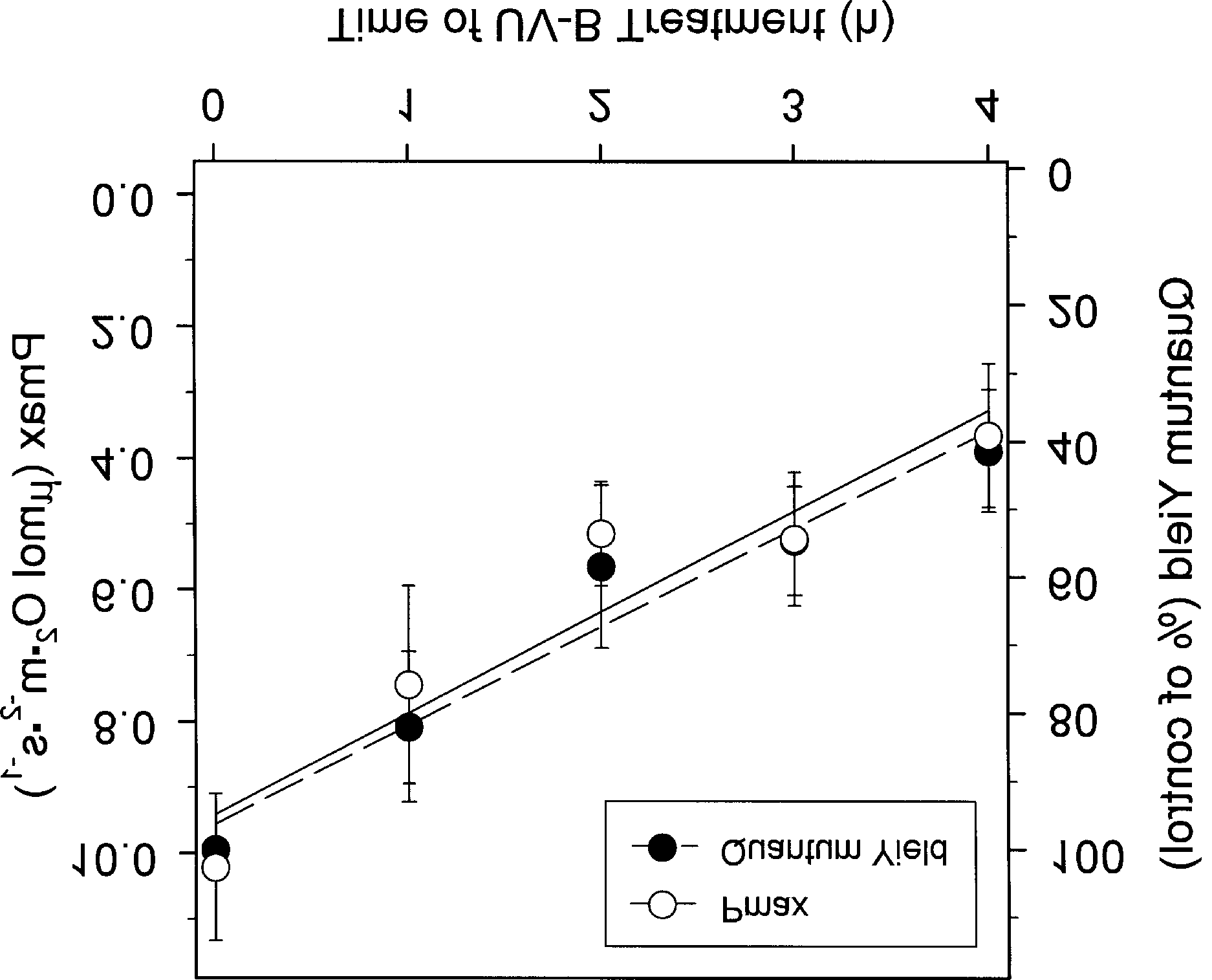 Photosynthetic Response and Protective Regulation to UV-B Radiation
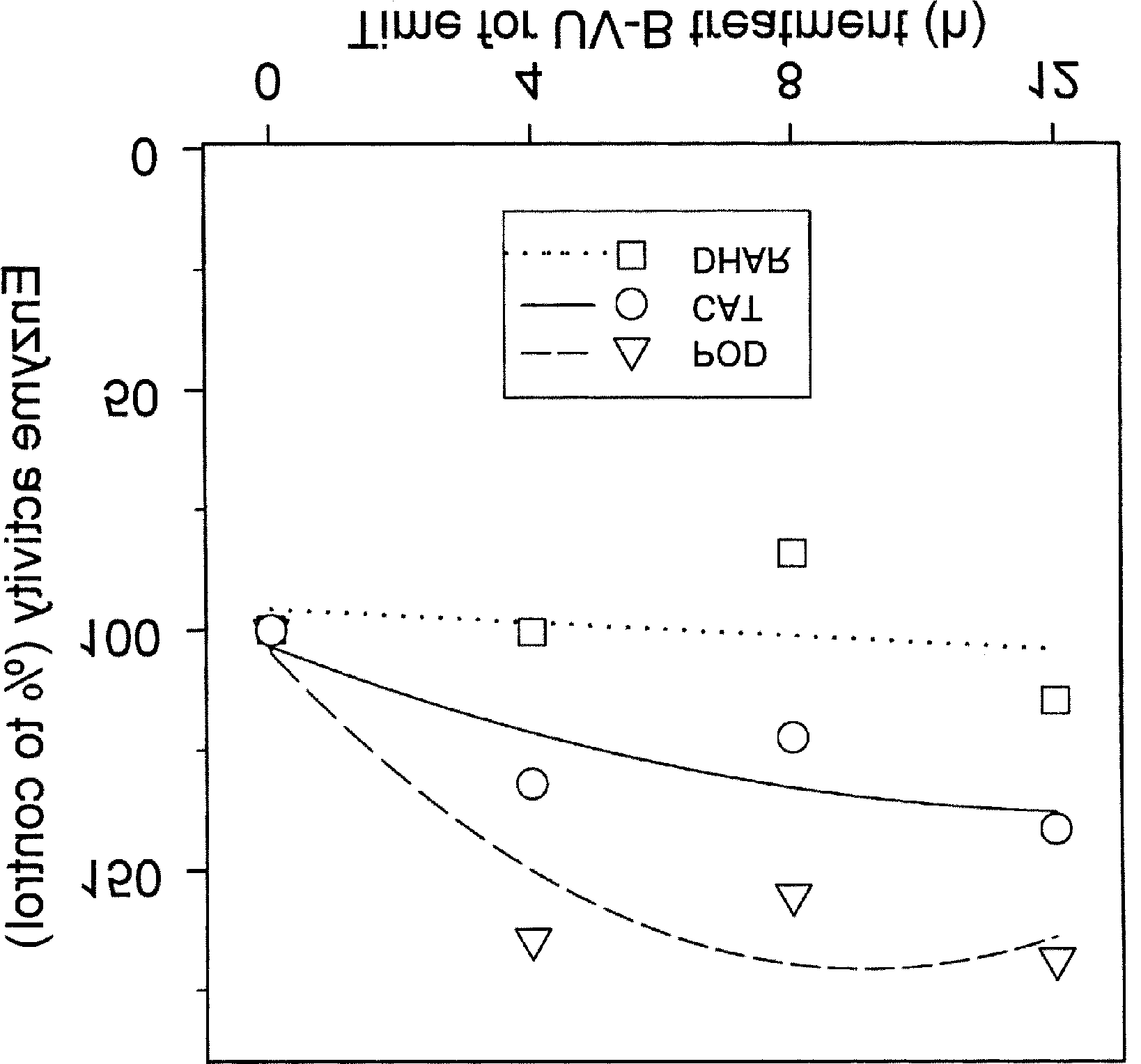
assayed according to Rao et al. [32]. CAT activity was determinedspectrophotometrically by the decrease of absorbance at 240 nmin a cuvette with 1 mL of reaction mixture containing 100 mMK-PO (pH 7.0), 40 µL of crude extract, and 10 mM H O , which
was added just prior to the initiation of reaction. GR was assayedin 1 mL of reaction mixture containing 100 mM K-PO (pH 7.8),
0.2 mM NADPH, 0.5 mM GSSG (oxidized glutathione), 2 mMEDTA, and 100 µL of crude extract by monitoring decrease ofabsorbance at 340 nm. The assay was initiated by the addition ofNADPH. POD activity was quantified by measuring the rate ofguajacol tetramerization shown as the increase in absorbance at470 nm in a cuvette containing 1 mL of reaction mixture of 100mM K-PO (pH 6.5), 16 mM guajacol, 10 mM H O , and 100 µL
of crude extract. The reaction was initiated by adding crudeextract and followed for 10 min.
Photosynthetic Response and Protective Regulation to UV-B Radiation
assayed according to Rao et al. [32]. CAT activity was determinedspectrophotometrically by the decrease of absorbance at 240 nmin a cuvette with 1 mL of reaction mixture containing 100 mMK-PO (pH 7.0), 40 µL of crude extract, and 10 mM H O , which
was added just prior to the initiation of reaction. GR was assayedin 1 mL of reaction mixture containing 100 mM K-PO (pH 7.8),
0.2 mM NADPH, 0.5 mM GSSG (oxidized glutathione), 2 mMEDTA, and 100 µL of crude extract by monitoring decrease ofabsorbance at 340 nm. The assay was initiated by the addition ofNADPH. POD activity was quantified by measuring the rate ofguajacol tetramerization shown as the increase in absorbance at470 nm in a cuvette containing 1 mL of reaction mixture of 100mM K-PO (pH 6.5), 16 mM guajacol, 10 mM H O , and 100 µL
of crude extract. The reaction was initiated by adding crudeextract and followed for 10 min.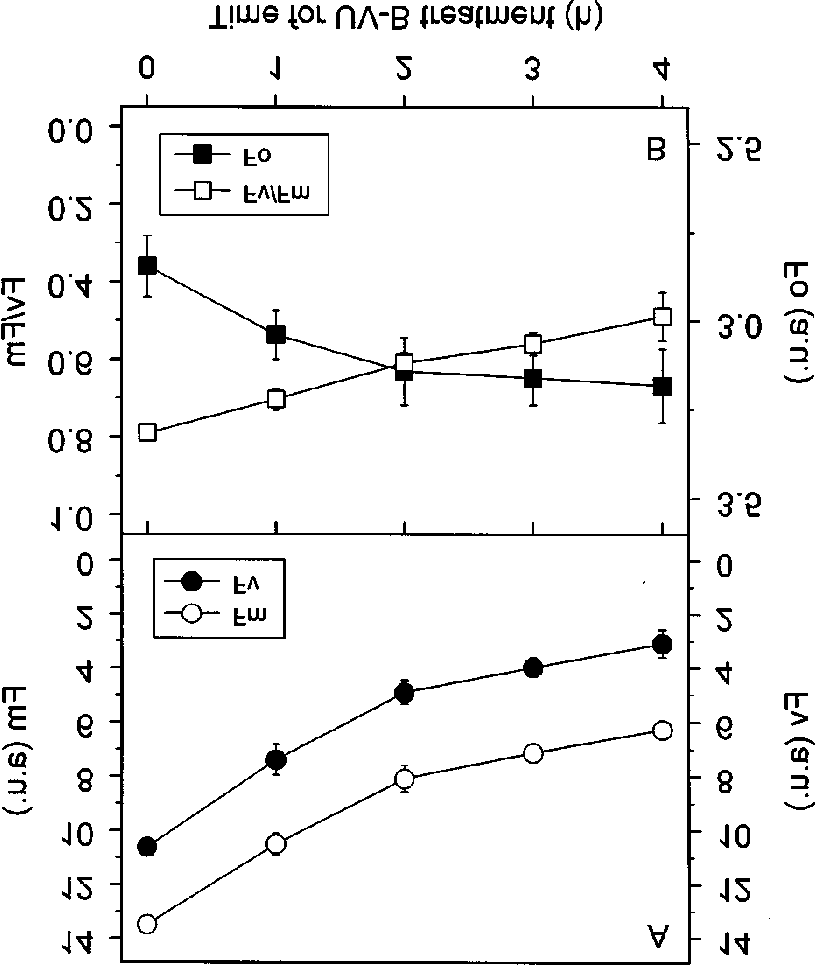
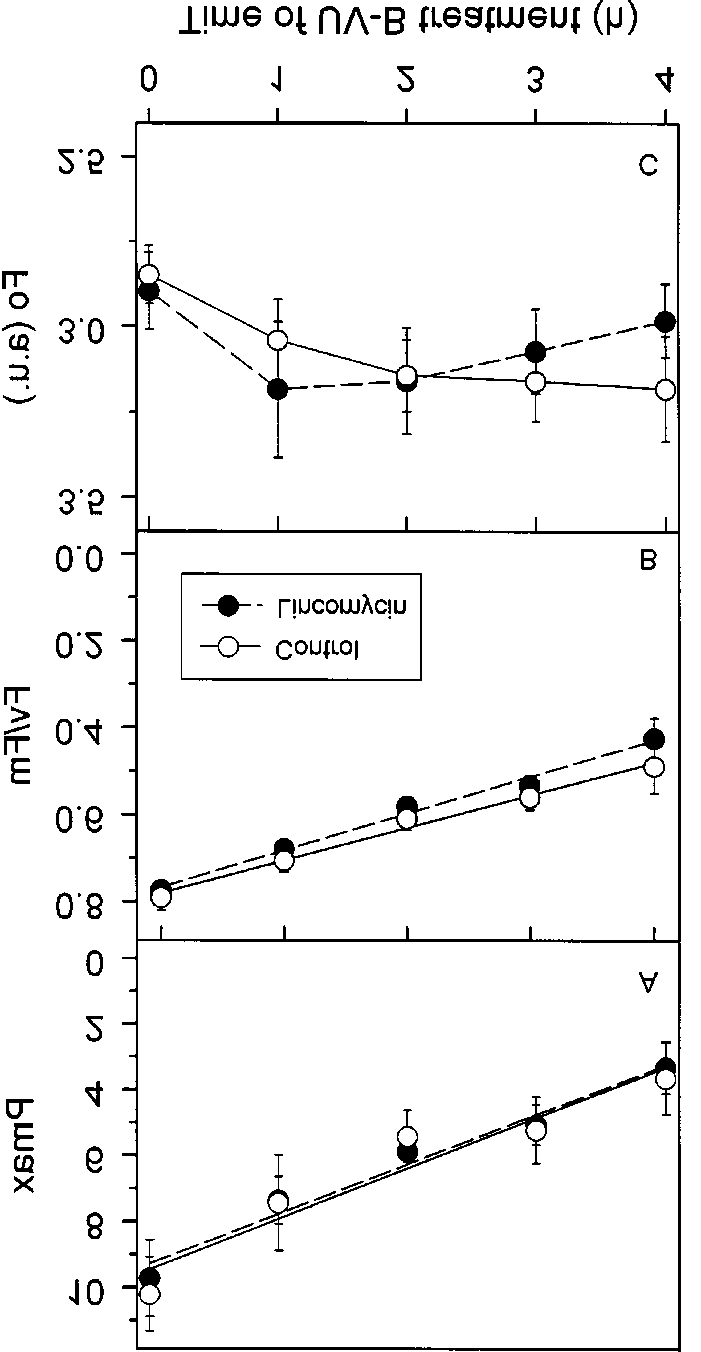
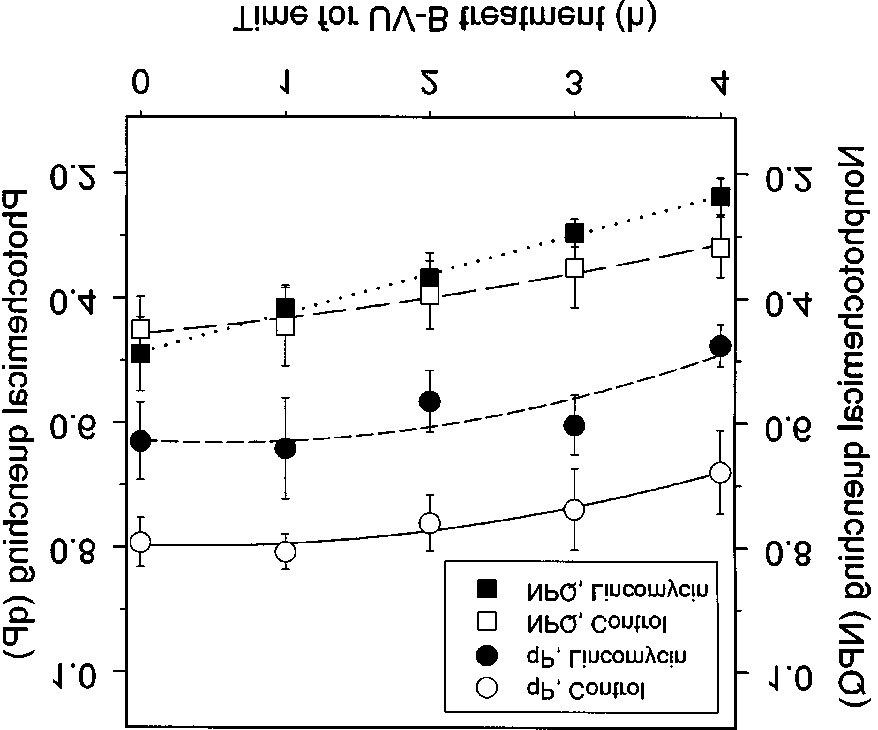 Figure 2. Changes in various Chl fluorescence parameters in theUV-B treated leaves: (A) the maximal (Fm) and variable (Fv)fluorescence, (B) the initial (Fo) and the maximal photochemicalefficiency of PSII (Fv/Fm). Fo, Fm, and Fv are given in arbitraryunits. Data presented are mean values ± S.E. for 5 measurements.
Figure 2. Changes in various Chl fluorescence parameters in theUV-B treated leaves: (A) the maximal (Fm) and variable (Fv)fluorescence, (B) the initial (Fo) and the maximal photochemicalefficiency of PSII (Fv/Fm). Fo, Fm, and Fv are given in arbitraryunits. Data presented are mean values ± S.E. for 5 measurements.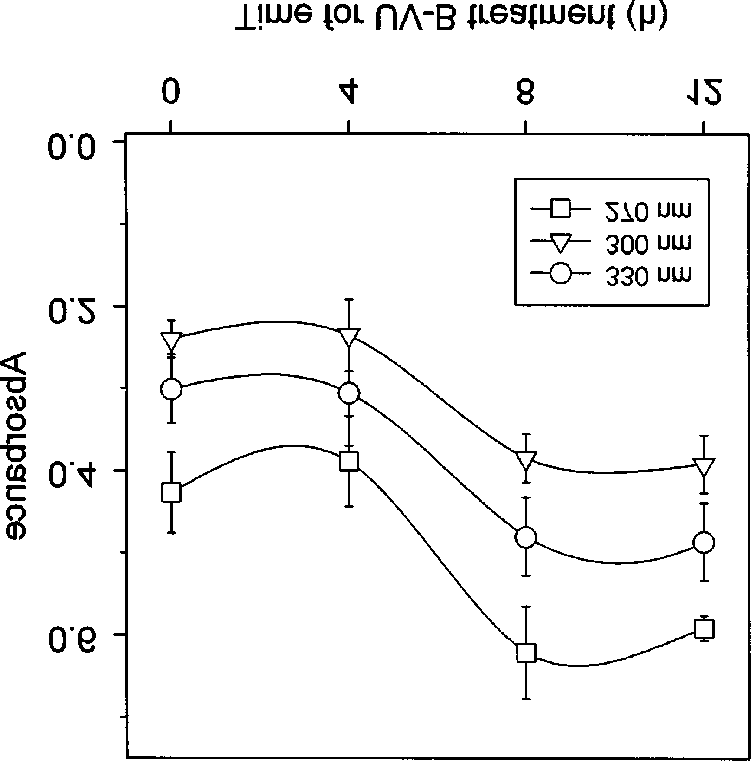
 Photosynthetic Response and Protective Regulation to UV-B Radiation
Table 1. Changes in the amount of photosynthetic pigments afterUV-B treatment.
Photosynthetic Response and Protective Regulation to UV-B Radiation
Table 1. Changes in the amount of photosynthetic pigments afterUV-B treatment.