February 2010 inventory log detail report
Cornell University Hospital for AnimalsInventory Log Detail Report for 2/1/2010 to 2/28/2010 MAIN_STORAGE ADMINISTRATION Inventory Log Detail Report Totals by Item.rptCornell University Hospital for AnimalsInventory Log Detail Report for 2/1/2010 to 2/28/2010 MAIN_STORAGE PHARMACY AMBULATORY PHARMACY Inventory Log Detail Report Totals by Item.rptCornell University Hospital for Ani

 Azacrown indoaniline dye as a sensing molecule in
optical sensors for the selective detection of Li+
Sung-Hoon Kim a,*, Jae-Woo Kim a, Jae-Ho Kim b, Kwang-Nak Koh c,
aDepartment of Dyeing and Finishing, College of Engineering, Kyungpook National University, Taegu 702-701, South Korea
bDepartment of Molecular Science and Technology, Ajou University, Suwon 442-749, South Korea
cSensor Technology Research Center, Kyungpook National University, Taegu 702-701, South Korea
Received 28 January 2000; received in revised form 18 February 2000; accepted 29 March 2000
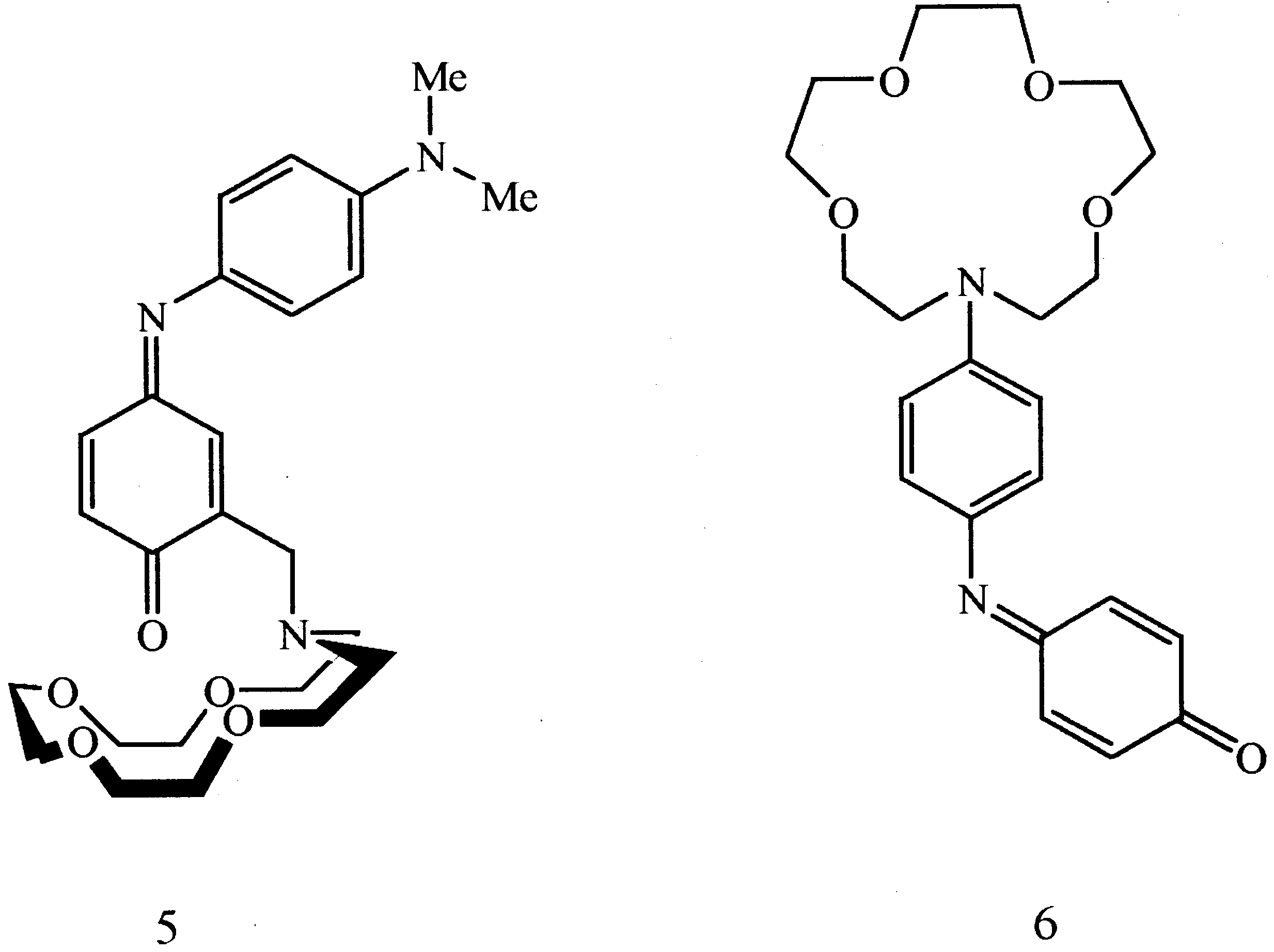
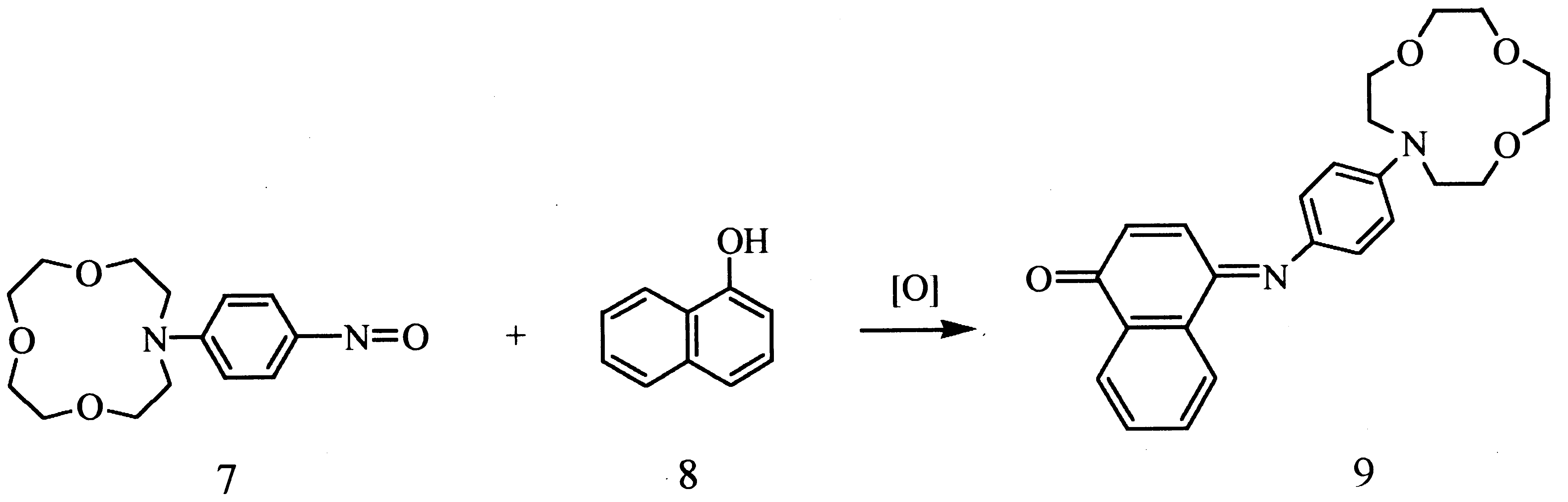
The complex formation of azacrown indoaniline (ACIA) dye 9 with Li+ and Na+ was investigated spectro-
photometrically. As the polarity of the solvent decreased, the complexation ability increased. ACIA 9 exhibits higher
Li+ selectivity than Na+ ion in CH2Cl2±CH3CN. The speci®c spectral response to Li+ ion by ACIA 9 indicates
potential suitability for optical sensor applications. # 2000 Published by Elsevier Science Ltd. All rights reserved.
Azacrown indoaniline dye as a sensing molecule in
optical sensors for the selective detection of Li+
Sung-Hoon Kim a,*, Jae-Woo Kim a, Jae-Ho Kim b, Kwang-Nak Koh c,
aDepartment of Dyeing and Finishing, College of Engineering, Kyungpook National University, Taegu 702-701, South Korea
bDepartment of Molecular Science and Technology, Ajou University, Suwon 442-749, South Korea
cSensor Technology Research Center, Kyungpook National University, Taegu 702-701, South Korea
Received 28 January 2000; received in revised form 18 February 2000; accepted 29 March 2000
The complex formation of azacrown indoaniline (ACIA) dye 9 with Li+ and Na+ was investigated spectro-
photometrically. As the polarity of the solvent decreased, the complexation ability increased. ACIA 9 exhibits higher
Li+ selectivity than Na+ ion in CH2Cl2±CH3CN. The speci®c spectral response to Li+ ion by ACIA 9 indicates
potential suitability for optical sensor applications. # 2000 Published by Elsevier Science Ltd. All rights reserved.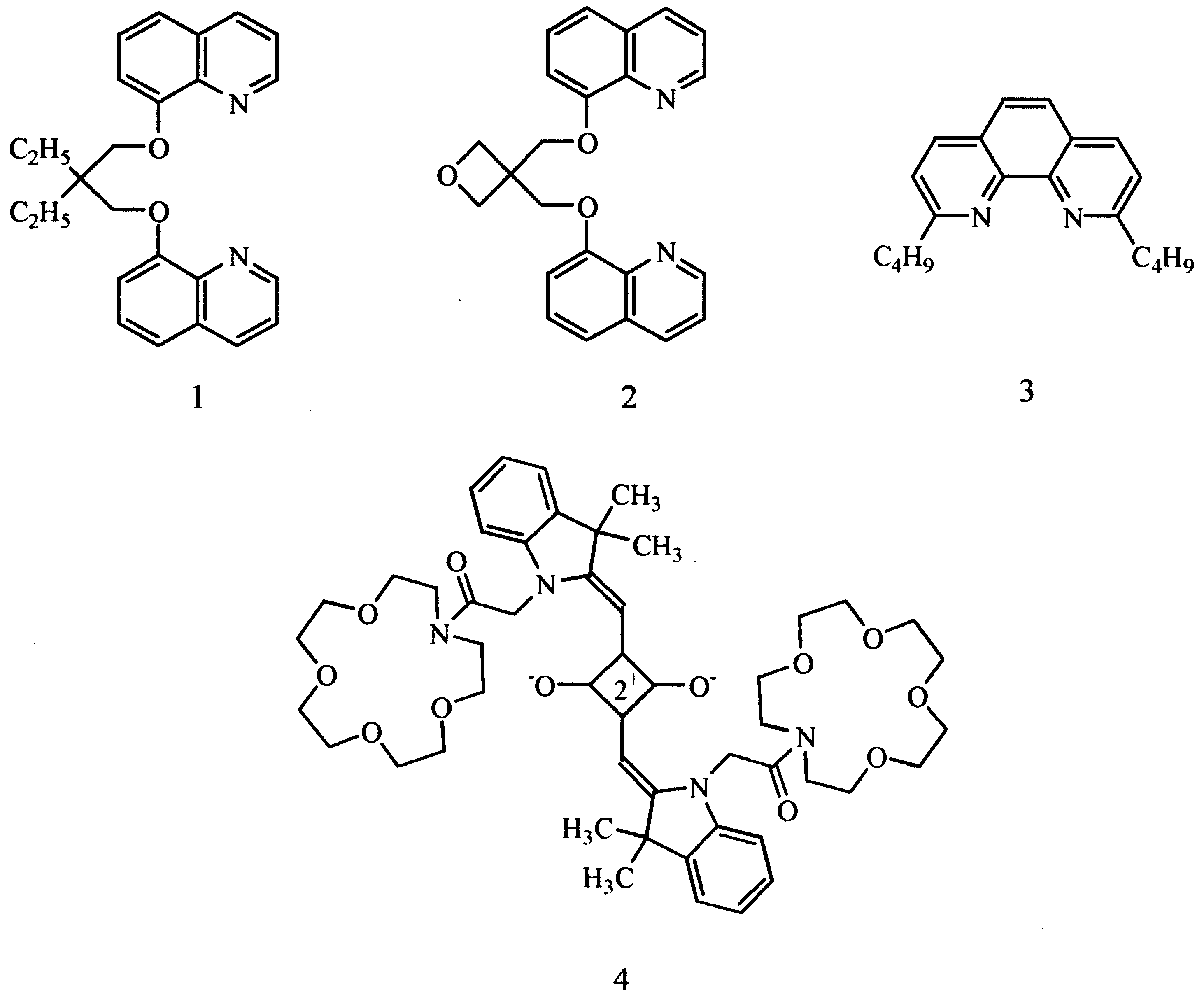
 S.-H. Kim et al. / Dyes and Pigments 46 (2000) 49±53
most of the commonly encountered metal ions. On
account of its high sensitivity, absorption spec-
troscopy is becoming increasingly important for
chemical trace detection. Sutherland et al. have
reported the use of a chromoionophore in an
optical sensor [13]. We are currently working on
the synthesis and study of newer derivatives of
indoaniline dyes, which can potentially yield a new
class of chromophores for the selective and quan-
titative detection of metal ions, both for biological
and environmental applications. Such indoaniline
dyes can be used as fat-soluble dyes. Dyes of this
class are not now used for textile coloration, but
they are applied in colour photography and also
serve as intermediates for sulfur dyes. We have
previously reported the synthesis and X-ray struc-
Acyclic Li+ ionophores 1±3, which are good
tural characterization of ACIA 9 carrying a
neutral carriers in Li+-selective electrodes, may
monoazacrown moiety [14]. Here we report the
also be applied to Li+ ¯uorometry [16,17]. We
metal complexation properties of this new ACIA
reported that the ¯uorescence emission intensity of
the azacrown squarylium dye 4 in CH2Cl2±
CH3CN (1:4/v:v) was signi®cantly enhanced by
the addition of lithium perchlorate. The presence
of calcium ion increased the ¯uorescence slightly,
but potassium and sodium had no eect. Chro-
All chemicals used were of analytical grade;
moionophores are based on the idea that the
LiClO4 and NaClO4 were obtained from Aldrich.
S.-H. Kim et al. / Dyes and Pigments 46 (2000) 49±53
most of the commonly encountered metal ions. On
account of its high sensitivity, absorption spec-
troscopy is becoming increasingly important for
chemical trace detection. Sutherland et al. have
reported the use of a chromoionophore in an
optical sensor [13]. We are currently working on
the synthesis and study of newer derivatives of
indoaniline dyes, which can potentially yield a new
class of chromophores for the selective and quan-
titative detection of metal ions, both for biological
and environmental applications. Such indoaniline
dyes can be used as fat-soluble dyes. Dyes of this
class are not now used for textile coloration, but
they are applied in colour photography and also
serve as intermediates for sulfur dyes. We have
previously reported the synthesis and X-ray struc-
Acyclic Li+ ionophores 1±3, which are good
tural characterization of ACIA 9 carrying a
neutral carriers in Li+-selective electrodes, may
monoazacrown moiety [14]. Here we report the
also be applied to Li+ ¯uorometry [16,17]. We
metal complexation properties of this new ACIA
reported that the ¯uorescence emission intensity of
the azacrown squarylium dye 4 in CH2Cl2±
CH3CN (1:4/v:v) was signi®cantly enhanced by
the addition of lithium perchlorate. The presence
of calcium ion increased the ¯uorescence slightly,
but potassium and sodium had no eect. Chro-
All chemicals used were of analytical grade;
moionophores are based on the idea that the
LiClO4 and NaClO4 were obtained from Aldrich.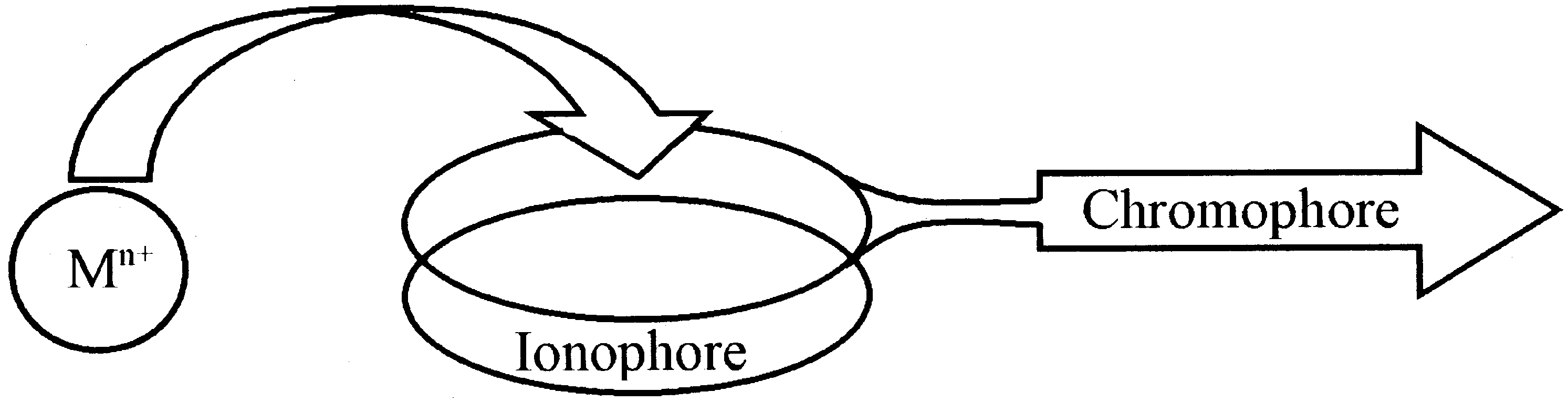
 S.-H. Kim et al. / Dyes and Pigments 46 (2000) 49±53
mine and aniline moieties associated with a
decrease in coplanarity. In order to determine the
dihedral angle between quinonimine and aniline
moiety, MM2 was performed. Geometry opti-
mization was carried out both before and after the
Fig. 1. Molecular construction of a chromoionophore.
S.-H. Kim et al. / Dyes and Pigments 46 (2000) 49±53
mine and aniline moieties associated with a
decrease in coplanarity. In order to determine the
dihedral angle between quinonimine and aniline
moiety, MM2 was performed. Geometry opti-
mization was carried out both before and after the
Fig. 1. Molecular construction of a chromoionophore.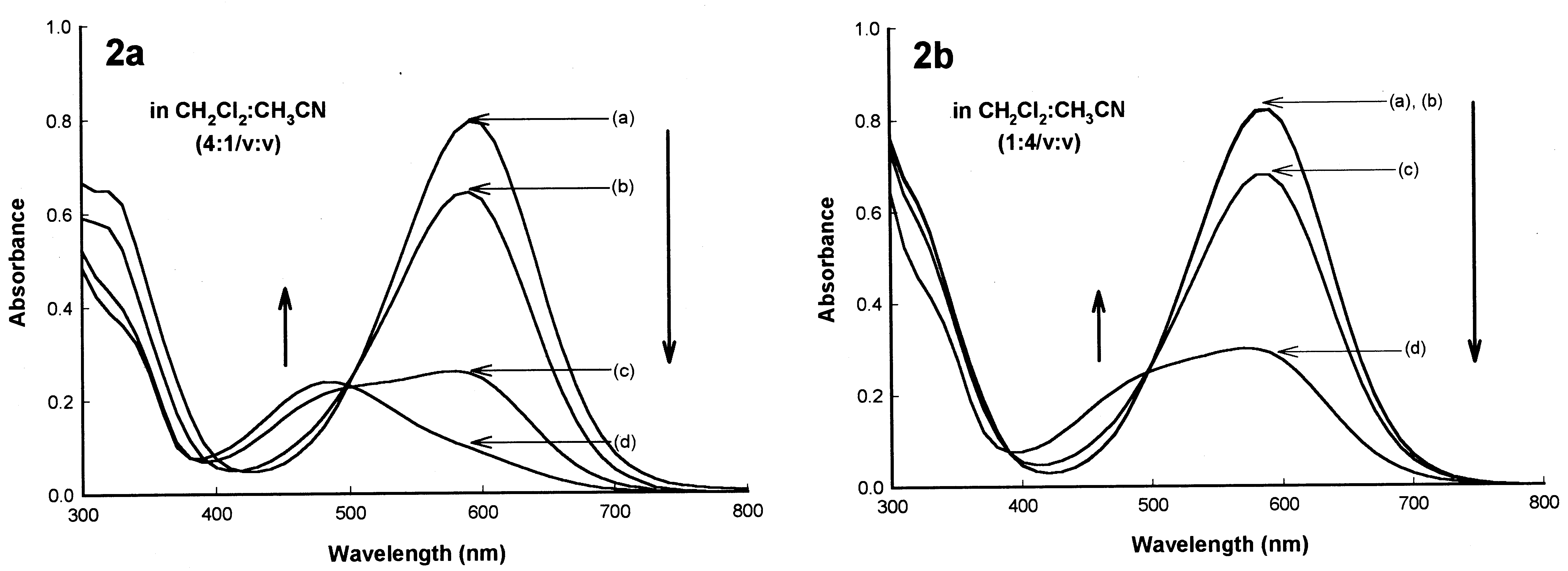
 S.-H. Kim et al. / Dyes and Pigments 46 (2000) 49±53
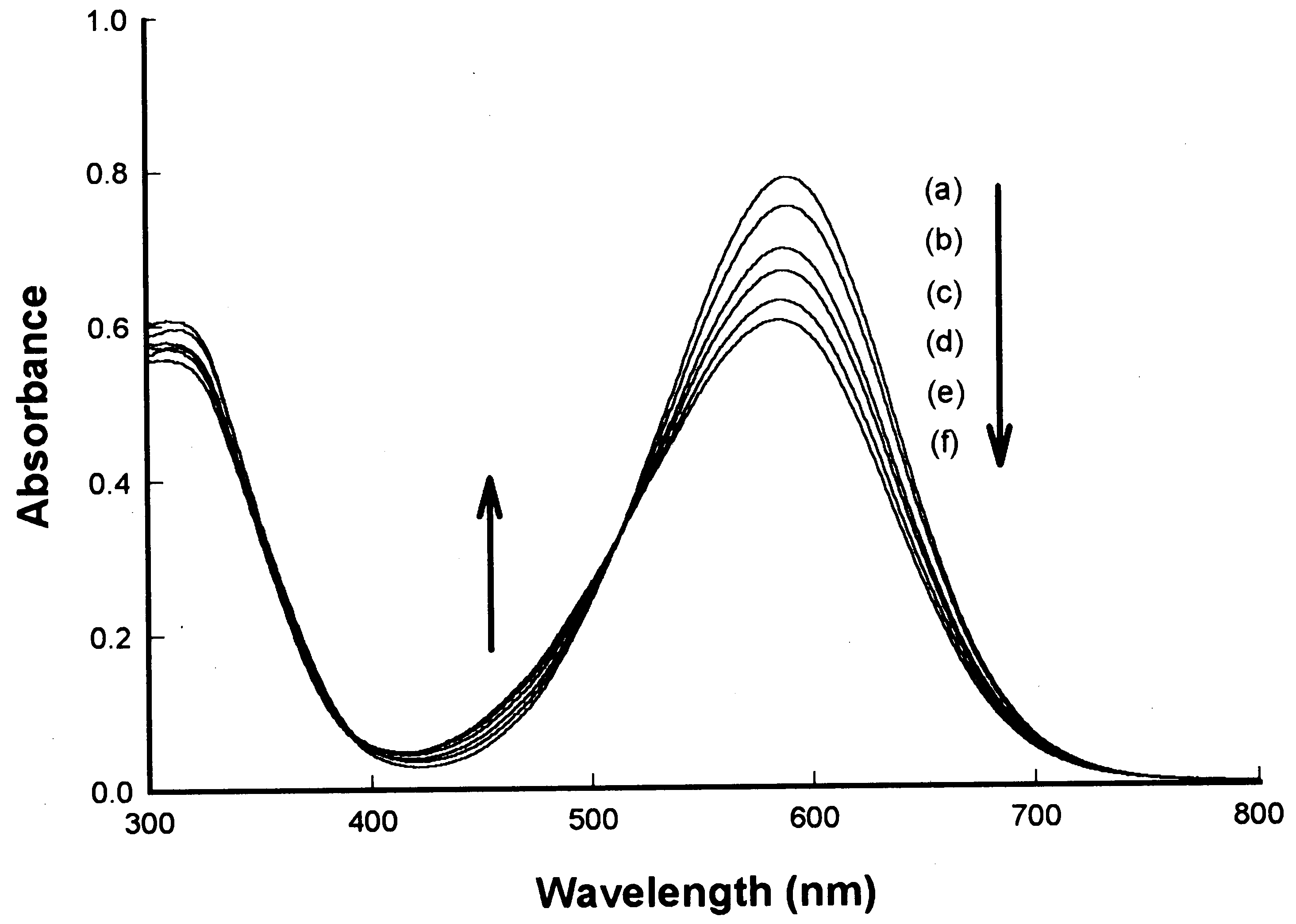
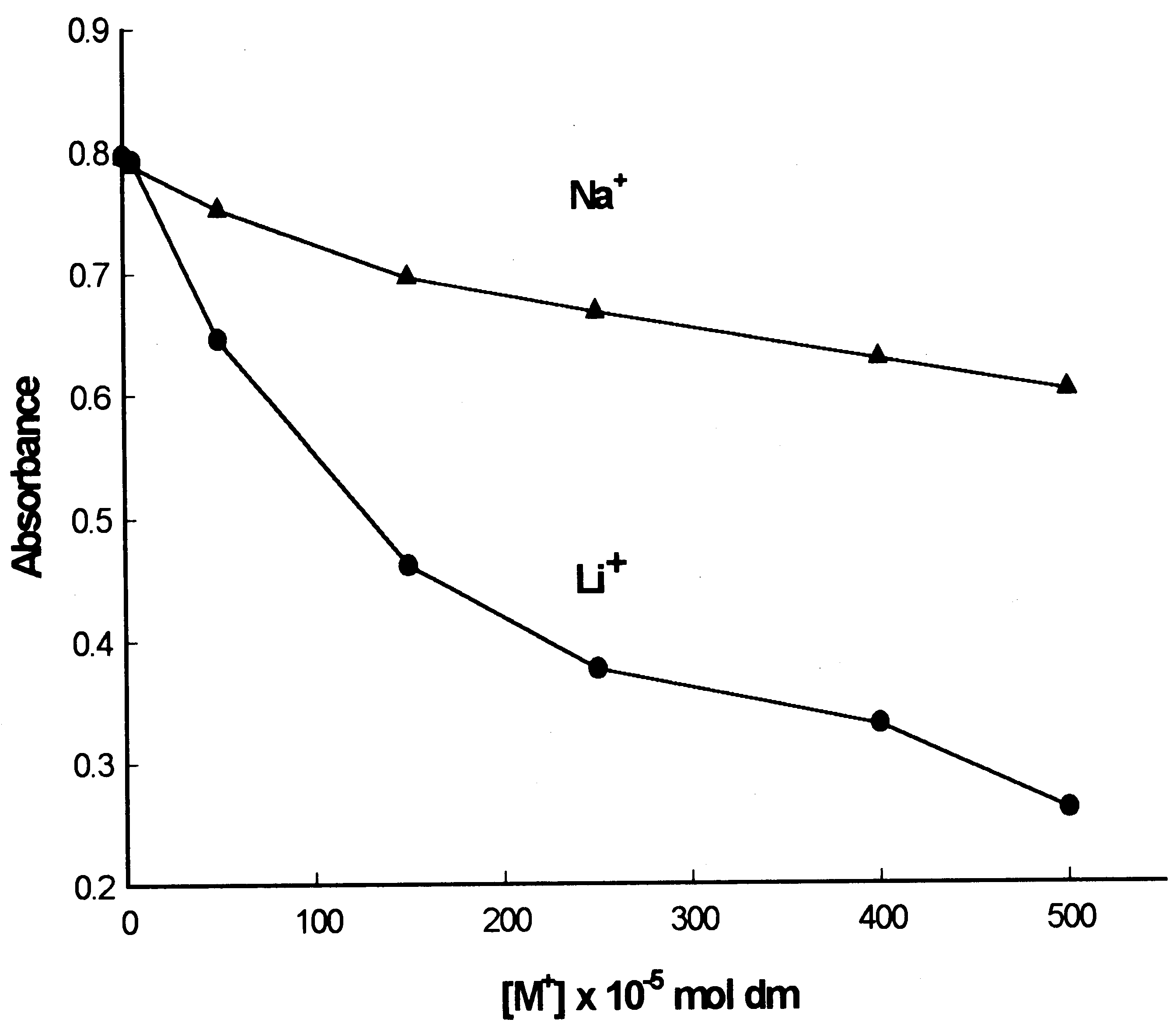
Fig. 2. Eect of Li+ concentration on the absorption of spectra of ACIA dye 9 (5Â10À5 M): [Li+] (a) 0, (b) 5Â10À4 M, (c) 5Â10À3 M,
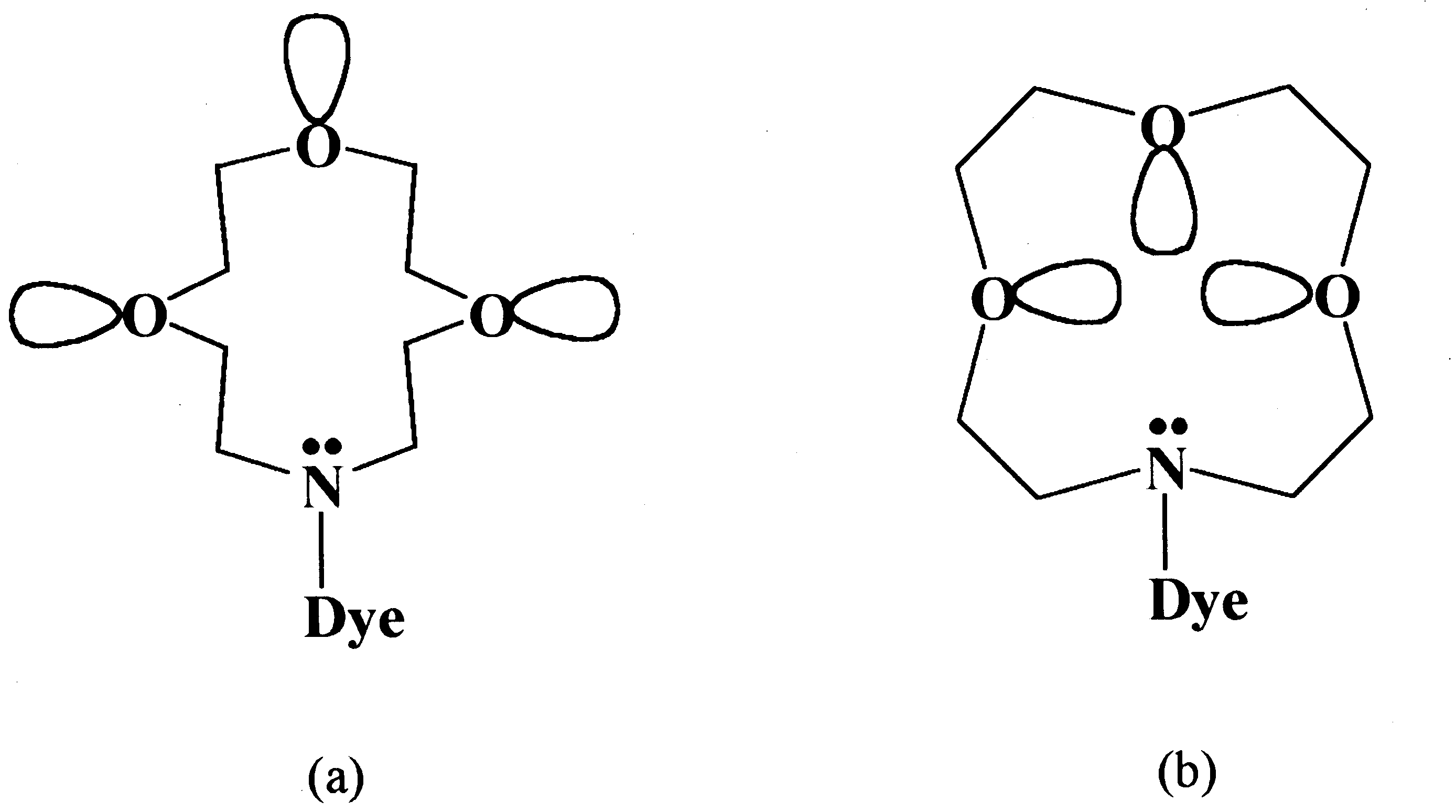
Fig. 3. Conceivable conformation of azacrown ring: (a) in
polar medium; (b) in non-polar medium.
S.-H. Kim et al. / Dyes and Pigments 46 (2000) 49±53
Fig. 2. Eect of Li+ concentration on the absorption of spectra of ACIA dye 9 (5Â10À5 M): [Li+] (a) 0, (b) 5Â10À4 M, (c) 5Â10À3 M,
Fig. 3. Conceivable conformation of azacrown ring: (a) in
polar medium; (b) in non-polar medium.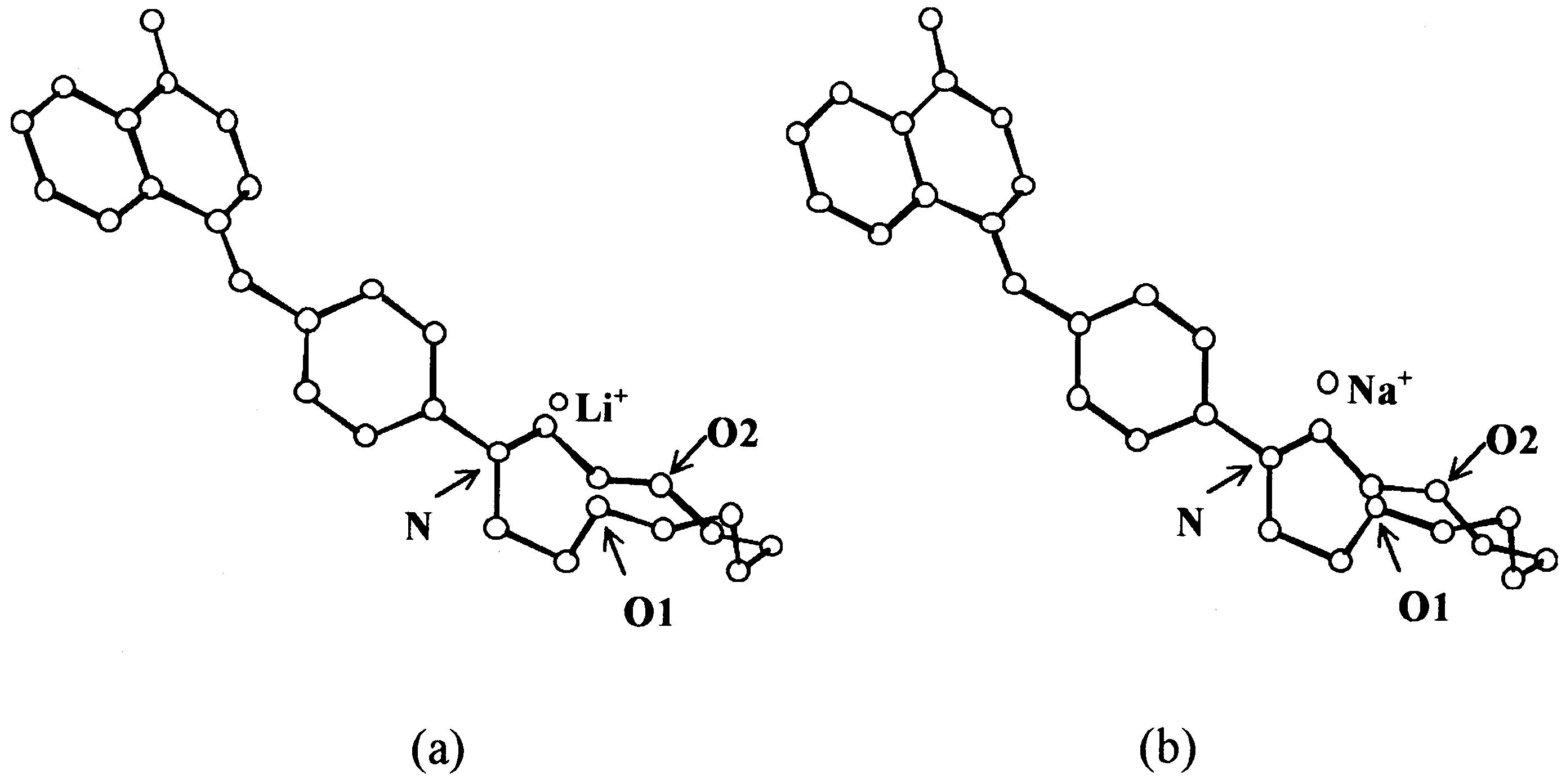
 S.-H. Kim et al. / Dyes and Pigments 46 (2000) 49±53
present system to selective Li+ recognition of the
self-assembled monolayer (SAM) on gold by sur-
[1] Bach RO. Lithium Ð current applications in science,
medicine and technology. New York: Wiley, 1985.
S.-H. Kim et al. / Dyes and Pigments 46 (2000) 49±53
present system to selective Li+ recognition of the
self-assembled monolayer (SAM) on gold by sur-
[1] Bach RO. Lithium Ð current applications in science,
medicine and technology. New York: Wiley, 1985.